Handling and Stability of Semaglutide: A Guide for Laboratory Researchers
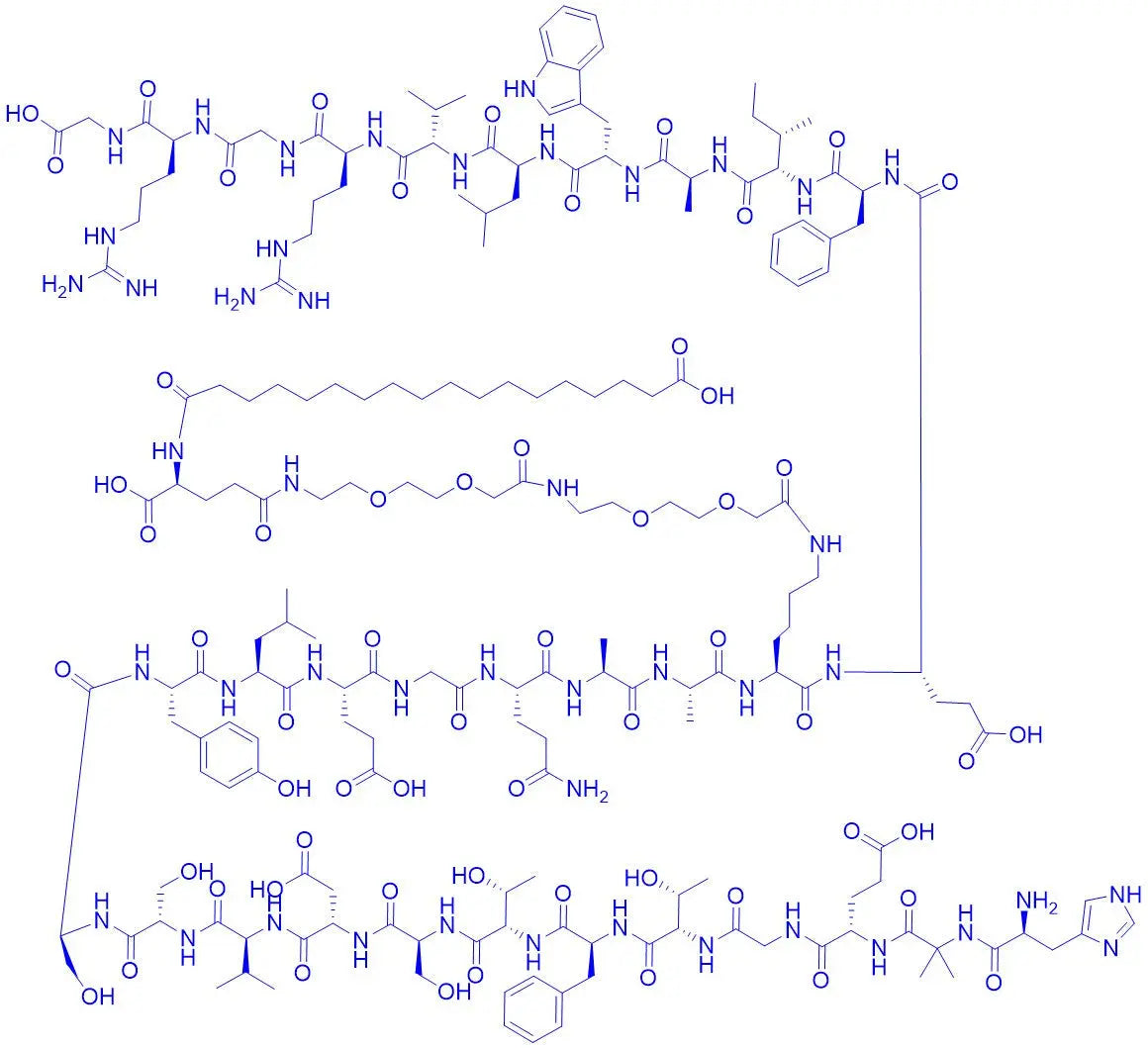
Semaglutide
The success and reproducibility of in vitro experiments are fundamentally dependent on the stability and integrity of the reagents used. For researchers studying the glucagon-like peptide-1 (GLP-1) receptor, obtaining consistent results requires a GLP-1 analogue that can withstand enzymatic degradation and remain active in experimental conditions. Semaglutide was specifically engineered to meet this need.
This article provides a technical guide for laboratory researchers on the structural features that contribute to Semaglutide's stability and outlines the best practices for its handling, reconstitution, and storage to ensure optimal performance in a research setting.
The Structural Basis for Semaglutide's Enhanced Stability
Semaglutide's superior stability compared to native GLP-1 is not accidental; it is the result of deliberate and ingenious chemical modifications. Understanding these features is key for any lab working with research peptides semaglutide.
-
DPP-4 Resistance: Native GLP-1 is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4). Semaglutide features a critical amino acid substitution at position 8, replacing Alanine with α-aminoisobutyric acid (Aib). This modification sterically hinders the DPP-4 enzyme, dramatically reducing its ability to cleave and inactivate the peptide (Knudsen & Lau, 2019).
-
Fatty Acid Acylation: A C18 fatty diacid chain is attached to the lysine residue at position 26 via a hydrophilic spacer. This modification promotes the peptide's ability to bind to albumin, a common protein in cell culture media, which protects it from degradation and clearance.
These engineered properties make Semaglutide a robust tool for experiments requiring sustained GLP-1 receptor activation.
-
CAS Number: 910463-68-2
-
Molecular Formula: C₁₈₇H₂₉₁N₄₅O₅₉
-
Molecular Weight: 4113.6 g/mol
From Powder to Solution: Reconstitution Best Practices
For maximum stability during shipping and storage, the compound is supplied as a lyophilized semaglutide peptide powder. Proper reconstitution is the first critical step to ensuring its experimental efficacy.
-
Equilibration: Before opening, allow the vial to come to room temperature to prevent condensation from introducing moisture.
-
Solvent Selection: Consult the technical data sheet provided by the supplier. Due to its modifications, Semaglutide may require a specific pH-adjusted buffer for optimal solubility. Do not assume it will readily dissolve in sterile water.
-
Reconstitution Technique: Using a sterile syringe, slowly introduce the recommended solvent into the vial, allowing it to run down the side of the glass. Gently swirl or roll the vial to dissolve the powder. Avoid vigorous shaking, as this can cause aggregation or degradation of the peptide.
Properly handling the semaglutide peptide at this initial stage is crucial for the success of all subsequent experiments.
Storage Protocols for Short and Long-Term Stability
Once reconstituted, the stability of the solution depends entirely on storage conditions.
-
Lyophilized Powder: Unopened vials of powder should be stored in a freezer at -20°C or colder, protected from light.
-
Reconstituted Solution: To prevent degradation from repeated freeze-thaw cycles, it is essential to aliquot the stock solution into smaller, single-use volumes. For short-term storage (a few days), these aliquots can be refrigerated at 4°C. For long-term storage (weeks to months), they must be stored frozen at -20°C or -80°C.
Following these protocols is essential when working with any semaglutide research chemical to ensure its potency is maintained throughout the course of a study.
Conclusion
The engineered stability of Semaglutide makes it an exceptional tool for the in vitro exploration of the GLP-1 receptor pathway. However, this stability can only be maintained through meticulous laboratory practice. Proper reconstitution and strict adherence to storage protocols are not merely suggestions but are integral parts of the experimental process itself.
The reliability of research chemicals semaglutide is directly tied to their initial purity and subsequent handling. By starting with a high-purity, independently verified compound and following these guidelines, researchers can have confidence in the integrity of their results.
Sources:
-
Knudsen, L. B., & Lau, J. (2019). The Discovery and Development of Liraglutide and Semaglutide. Frontiers in Endocrinology, 10, 155.
-
National Center for Biotechnology Information (2025). PubChem Compound Summary for CID 56843331, Semaglutide. Retrieved July 14, 2025 from https://pubchem.ncbi.nlm.nih.gov/compound/Semaglutide.
-
Heppner, K. M., & Perez-Tilve, D. (2015). GLP-1-based therapeutics: simultaneously addressing glycemic control and body weight. Reviews in Endocrine and Metabolic Disorders, 16(1), 37–49. (This source provides background on the mechanisms of GLP-1 analogues).
Disclaimer
All products available on this website are sold for laboratory research and in vitro purposes only. They are not for use in humans or animals and are not intended for any therapeutic or diagnostic application. By purchasing from Mindful Research, the customer acknowledges the known and unknown risks associated with the handling and use of these chemical compounds and confirms they are a qualified professional (e.g., a researcher, scientist, or technician) who will use these products in a properly equipped facility in accordance with all applicable laws and regulations.
The statements made within this website have not been evaluated by the US Food and Drug Administration. The statements and the products of this company are not intended to diagnose, treat, cure or prevent any disease.
Mindful Research is a chemical supplier. Mindful Research is not a compounding pharmacy or chemical compounding facility as defined under 503A of the Federal Food, Drug, and Cosmetic act. Mindful Research is not an outsourcing facility as defined under 503B of the Federal Food, Drug, and Cosmetic act.
